Sandoz Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SANDOZ, and what generic alternatives to SANDOZ drugs are available?
SANDOZ has four hundred and eighty-six approved drugs.
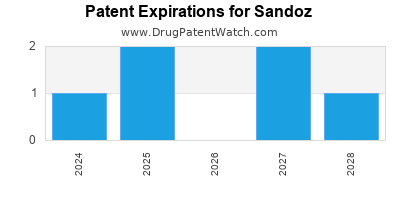
There are nine US patents protecting SANDOZ drugs. There are thirty-three tentative approvals on SANDOZ drugs.
There are one hundred and three patent family members on SANDOZ drugs in thirty-four countries and five hundred supplementary protection certificates in seventeen countries.
Summary for Sandoz
| International Patents: | 103 |
| US Patents: | 9 |
| Tradenames: | 320 |
| Ingredients: | 298 |
| NDAs: | 486 |
| Patent Litigation for Sandoz: | See patent lawsuits for Sandoz |
| PTAB Cases with Sandoz as petitioner: | See PTAB cases with Sandoz as petitioner |
Drugs and US Patents for Sandoz
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sandoz | GRANISETRON HYDROCHLORIDE | granisetron hydrochloride | INJECTABLE;INJECTION | 078531-002 | Apr 30, 2009 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sandoz | LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE | hydrochlorothiazide; losartan potassium | TABLET;ORAL | 077948-003 | Aug 19, 2010 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sandoz | VIVELLE-DOT | estradiol | SYSTEM;TRANSDERMAL | 020538-009 | May 3, 2002 | AB1 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sandoz | QUETIAPINE FUMARATE | quetiapine fumarate | TABLET;ORAL | 078679-002 | Dec 14, 2012 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sandoz | TREPROSTINIL | treprostinil | INJECTABLE;IV (INFUSION), SUBCUTANEOUS | 203649-001 | Nov 30, 2017 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Sandoz
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sandoz | VIVELLE | estradiol | SYSTEM;TRANSDERMAL | 020323-002 | Oct 28, 1994 | 5,300,291 | ⤷ Try a Trial |
| Sandoz | AZOPT | brinzolamide | SUSPENSION/DROPS;OPHTHALMIC | 020816-001 | Apr 1, 1998 | 5,378,703*PED | ⤷ Try a Trial |
| Sandoz | EXELON | rivastigmine | FILM, EXTENDED RELEASE;TRANSDERMAL | 022083-005 | Aug 31, 2012 | 6,335,031 | ⤷ Try a Trial |
| Sandoz | ZOFRAN | ondansetron hydrochloride | TABLET;ORAL | 020103-003 | Aug 27, 1999 | 5,344,658*PED | ⤷ Try a Trial |
| Sandoz | FOCALIN | dexmethylphenidate hydrochloride | TABLET;ORAL | 021278-003 | Nov 13, 2001 | 6,528,530 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for SANDOZ drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Injection | 100 mg/mL, 2.5 mL vials | ➤ Subscribe | 2007-09-24 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2015-06-03 |
| ➤ Subscribe | Oral Solution | 4 mg/5 mL | ➤ Subscribe | 2004-12-20 |
| ➤ Subscribe | Extended-release Capsules | 5mg, 10mg and 20 mg | ➤ Subscribe | 2007-03-30 |
| ➤ Subscribe | Injection | 0.05 mg/mL, 100 mL vial | ➤ Subscribe | 2008-08-29 |
| ➤ Subscribe | Extended-release Capsule | 40 mg | ➤ Subscribe | 2010-12-20 |
| ➤ Subscribe | Otic Suspension | 0.3%/0.1% | ➤ Subscribe | 2012-07-31 |
| ➤ Subscribe | Extended-release capsules | 35 mg | ➤ Subscribe | 2011-09-29 |
| ➤ Subscribe | Tablets | 2.5 mg | ➤ Subscribe | 2004-07-27 |
| ➤ Subscribe | Extended-release Capsules | 20 mg, 30 mg and 40 mg | ➤ Subscribe | 2006-08-21 |
| ➤ Subscribe | Capsules | 5 mg/40 mg and 10 mg/40 mg | ➤ Subscribe | 2006-11-17 |
| ➤ Subscribe | Capsules | 1.5 mg, 3 mg, 4.5 mg and 6 mg | ➤ Subscribe | 2004-04-21 |
| ➤ Subscribe | Transdermal System Extended-release | 13.3 mg/24 hr | ➤ Subscribe | 2013-01-22 |
| ➤ Subscribe | Injection | 1 mg/mL, 50 mL vials | ➤ Subscribe | 2011-12-16 |
| ➤ Subscribe | Extended-release Capsules | 15 mg | ➤ Subscribe | 2007-05-14 |
| ➤ Subscribe | For Injection | 250 mg/vial | ➤ Subscribe | 2009-09-01 |
| ➤ Subscribe | Extended-release Capsule | 30 mg | ➤ Subscribe | 2010-12-15 |
| ➤ Subscribe | Ophthalmic Solution | 0.00% | ➤ Subscribe | 2009-02-19 |
| ➤ Subscribe | Extended-release capsules | 25 mg | ➤ Subscribe | 2011-09-30 |
| ➤ Subscribe | Extended-release Tablets | 80 mg | ➤ Subscribe | 2007-03-15 |
| ➤ Subscribe | Extended-release Capsules | 10 mg | ➤ Subscribe | 2007-05-21 |
| ➤ Subscribe | Tablets | 5 mg and 10 mg | ➤ Subscribe | 2004-05-27 |
| ➤ Subscribe | Capsules | 2.5 mg/10 mg, 5 mg/10 mg, 5 mg/20 mg and 10 mg/20 mg | ➤ Subscribe | 2004-06-09 |
| ➤ Subscribe | Ophthalmic Emulsion | 0.05% | ➤ Subscribe | 2014-05-01 |
| ➤ Subscribe | Oral Solution | 2 mg/mL | ➤ Subscribe | 2004-11-05 |
International Patents for Sandoz Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| World Intellectual Property Organization (WIPO) | 03026671 | ⤷ Try a Trial |
| Argentina | 045670 | ⤷ Try a Trial |
| Taiwan | 200530250 | ⤷ Try a Trial |
| Australia | 2004271731 | ⤷ Try a Trial |
| South Korea | 20060087568 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Sandoz Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1853250 | PA2014022 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: PACLITAXELUM; REGISTRATION NO/DATE: EU/1/07/428/001-002 20131220 |
| 1539166 | 2013C/064 | Belgium | ⤷ Try a Trial | PRODUCT NAME: DEXTROMETHORPHANE OU UN SEL, PRCURSEUR DE DERIVE PHARMACEUTIQUEMENT ACCEPTABLE, PAR EXEMPLE LE BROMHYDRURE DE DEXTROMETORPHANE ET EN PARTICULIER LE BROMHYDRURE DE DEXTROMETROPHANE MONHYDRATE ET QUINIDINE OU UN SEL,....; AUTHORISATION NUMBER AND DATE: EU/1/13/833 20130626 |
| 0480717 | C990009 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: MONTELUKASTUM, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, IN HET BIJZONDER NATRII MONTELUKASTUM; NATL REGISTRATION NO/DATE: RVG 23164 AND RVG 23165 19981103; FIRST REGISTRATION: FI 12766 AND 12767 19970825 |
| 2316456 | 65/2017 | Austria | ⤷ Try a Trial | PRODUCT NAME: NALTREXON ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON, INSBESONDERE NALTREXONHYDROCHLORID, UND BUPROPION ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON, INSBESONDERE BUPROPIONHYDROCHLORID; REGISTRATION NO/DATE: EU/1/14/988 (MITTEILUNG) 20150330 |
| 1746976 | 300885 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: IRINOTECANSUCROSOFAATZOUT; REGISTRATION NO/DATE: EU/1/16/1130 20161018 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.